Tracey Petryshen, PhD

Contact
petryshen@chgr.mgh.harvard.eduBiography
Dr. Petryshen received a Ph.D. in Medical Genetics from the University of Calgary in 2001 for her thesis work involving genetic linkage and association studies of dyslexia. She subsequently completed a postdoctoral fellowship investigating the genetic basis of neuropsychiatric disorders with Dr. Pamela Sklar at the Broad Institute of Harvard and MIT. In 2005, Dr. Petryshen was appointed Instructor in Psychiatry at Harvard Medical School. Her research laboratory is located in the Psychiatric and Neurodevelopmental Genetics Unit in the Center for Human Genetic Research at Massachusetts General Hospital. Dr. Petryshen is PI of the Boston CIDAR Genetics Core. She also directs the Behavioral Neurogenetics Laboratory of the Stanley Center for Psychiatric Research at the Broad Institute.Research Summary
Dr. Petryshen's research focuses on the identification of genes that confer risk of multi-factorial psychiatric and behavioral disorders, specifically schizophrenia and bipolar disorder. Her group uses both patient genetic studies, and mouse behavioral and neuromolecular studies, to uncover risk genes and neural circuits underlying the disorders and to examine their roles in disease pathophysiology. The approach to utilize both patient and mouse genetic studies has two complementary directions: 1) patient studies are performed to identify candidate disease genes that may be examined in mouse using powerful genomic tools to determine the effect of gene expression and allelic changes at both the phenotypic and cellular levels, and 2) mouse behavioral and neurophysiological models of psychiatric disorders are examined to identify candidate disease genes whose homologs can be examined for genetic association in clinical populations.Dr. Petryshen has led and participated in numerous studies to identify candidate loci and genes for schizophrenia and bipolar disorder encompassing linkage, haplotype-based gene association, population genetic, and microarray mRNA expression methods. Among other findings, these studies have obtained evidence for association between schizophrenia and the neuregulin 1 (NRG1) gene, as well as altered NRG1 expression levels in patients (Petryshen et al. 2005a), which led to a review article proposing a model of neuregulin 1 function in schizophrenia pathogenesis (Scolnick et al. 2006). In addition, a positional candidate gene study of a schizophrenia locus on chromosome 5q (Sklar et al. 2004) detected association between schizophrenia and GABAA receptor subunit genes, as well as correlations between GABAA gene haplotypes and expression levels of synaptic and vesicle-associated genes (Petryshen et al. 2005b). These findings provide support for the longstanding hypothesis of GABA system involvement in schizophrenia, and suggest a mechanism that involves key neuronal functions. An ongoing project in Dr. Petryshen's laboratory aims to identify mouse chromosomal regions containing genes that regulate prepulse inhibition of startle (PPI), a fundamental neurophysiological mechanism that underlies schizophrenia, as a means of providing insight into the genetics of schizophrenia. More recently, Dr. Petryshen established the Behavioral Neurogenetics Laboratory in the Stanley Center at the Broad Institute, which aims to characterize putative psychiatric risk genes identified from patient studies, and to examine the therapeutic potential of novel small molecules, through mouse behavioral and neurobiological studies.
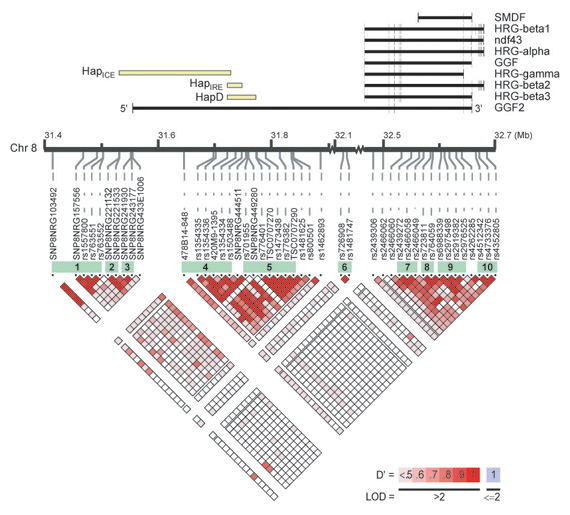
The linkage disequilibrium and haplotype structure of the neuregulin 1 gene (NRG1) implicated in schizophrenia risk. NRG1 consists of at least 15 transcript isoforms, of which nine major isoforms are shown at the top of the illustration. The location of previously reported risk haplotypes within the NRG1 gene, HapICE, HapIRE, and HapD, are indicated relative to their position within the NRG1 genomic region. The linkage disequilibrium (LD) structure spanning the NRG1 region is indicated by the pseudo-color matrix, with red indicating strong LD between a pair of chromosomal markers. Markers in strong LD are transmitted together on a chromosome, and thus can be used as proxies when searching for markers associated with disease risk. Haplotype blocks consisting of markers in strong LD are shown in green. Graphical representation created by Locusview 2.0 software (Petryshen and Kirby, unpublished software; http://www.broad.mit.edu/mpg/locusview). See Petryshen et al. Mol Psychiatry 2005;10:366-374, Figure 2.
Selected Publications
- Scolnick EM, Petryshen T, Sklar P. Schizophrenia: Do the genetics and neurobiology of neuregulin provide a pathogenesis model? Harv Rev Psychiatry 2006; 14:64-77.
- Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry 2005b;10:1074-88,1057.
- Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, Medeiros HM, Carvalho C, Macedo A, Dourado A, Valente J, Ferreira CP, Patterson NJ, Azevedo MH, Daly MJ, Pato CN, Pato MT, Sklar P. Support for involvment of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry 2005a;10:366-374.
- Sklar P, Pato CN, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Verner A, Hudson TJ, Morley CP, Kennedy JL, Azevedo MH, Lander E, Daly MJ, Pato MT. Genome-wide scan in Portuguese island families Identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry 2004; 9:213-8.
