Scientific Details
Note: The information on this page is primarily directed towards researchers and clinicians. If you are interested in learning more about participating in this study, please view the Study Participation section of the website.
Since this is a large, complex center grant, which includes four projects and four cores, we present the overall organization of the grant in the figure and descriptions below (PI = Principal Investigator). Each participant enrolled in this project will have assessments completed by Core 1 (clinical assessments), Core 2 (genetics), Core 3 (imaging) and each of the projects. Information will be shared between the projects and the cores.
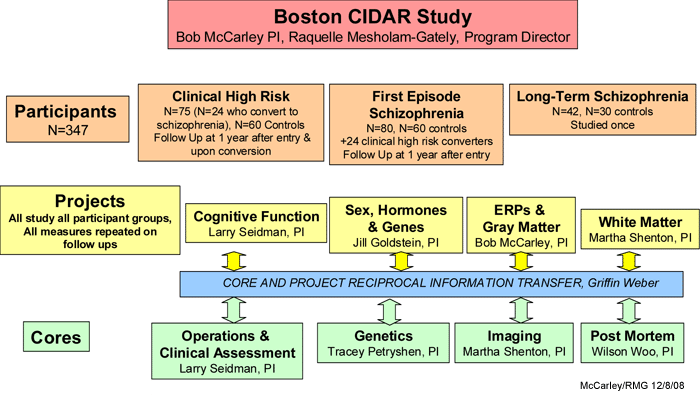
Projects
Project 1. "Functional anatomy of neurocognitive deterioration in schizophrenia" (i.e., "cognitive function"), L.
Seidman, Ph.D., PI. Uses neuropsychological, fMRI and structural MRI evaluations.
- Larry Seidman, PhD, PI
- Ariel Brown, MA, Neuroimaging Assistant
- Ann Cousins, MS, APRN, BC, Head of Training for Clinical Ratings, Clinical Rater, Investigator
- Michelle Friedman-Yakoobian, PhD, Clinical Rater, Investigator
- Shiva Gautam, PhD, Statistician
- Anthony Giuliano, PhD, Prodrome Project Director, Investigator
- Nikos Makris, MD, PhD, Neuroimaging / Investigator
- Raquelle Mesholam-Gately, PhD, CIDAR Program Director, Investigator
- Heidi Thermenos, PhD, Neuroimaging Coordinator, Investigator
- Joanne Wojcik, MS, APRN, BC, First Episode Project Director / Investigator
The central theme of this project, "Functional anatomy of neurocognitive deterioration in schizophrenia", is that schizophrenia is characterized by decline in neuropsychological and brain function, as well as alterations in brain structure much of it occurring by the first episode. In a refinement of the Kraepelinian view, we hypothesize that a substantial proportion of decline, particularly neuropsychological decline, occurs prior to the onset of schizophrenia. The main goal of this project is to understand the evolution of neuropsychological deficits from clinical high risk to long-term/persistent schizophrenia, and to map its associated neural substrates. This study is designed to identify neurobiological processes and functional outcomes of participants considered clinical high risk and those in the first episode of schizophrenia within a longitudinal, developmental framework that includes long-term schizophrenia to index the "end stage" of progression. Such data are crucial to inform development of optimal early intervention and prevention approaches.
Project 2. "Hormones, memory & sex effects in illness progression in schizophrenia", J. Goldstein, Ph.D., PI.
Targets hormones, genes and sex differences
- Jill Goldstein, PhD, PI
- Anne Klibanski, MD, Investigator
- Jean Frazier, M.D. , Investigator
- Wilson Woo, MD, Ph.D., Investigator
- Larry Seidman, Ph.D, Investigator
- Nikos Makris, MD, Ph.D., Investigator
- Brandon Abbs, Ph.D. Postdoctoral fellow
- Laura Holsen, Ph.D. Imaging coordinator
- Lauren Ogden, BA, Research Assistant
An investigation of the role of hormones and genes in understanding the trajectory of sex differences in illness progression is important in that it may provide insights into the timing of potential differential hormonal interventions for men and women with schizophrenia. Our underlying premise is based on animal and human literature demonstrating the positive effects of estradiol (and negative effects of glucocorticoids) on declarative memory performance, in part through their impact on brain-derived neurotrophic growth factor (BDNF)- and GABA-mediated synaptic connectivity on long-term potentiation in two sexually dimorphic brain regions, hippocampus and prefrontal cortex. We will use functional and structural magnetic resonance imaging (fMRI & sMRI), hormonal evaluations, molecular genetics, and postmortem tissue analyses to test our hypotheses regarding sex differences in illness progression. Individuals will be recruited over 5 years to undergo an fMRI paradigm of memory and working memory function and hormonal evaluations in response to memory tasks. We will characterize sex differences in the associations between deficits in brain activity in hippocampus (HIPP), dorsolateral prefrontal cortex and anterior cingulate gyrus in response to a memory task, structural abnormalities in these regions, and neuroendocrine dysfunction associated with these brain deficits in schizophrenia compared with healthy controls. Further, we will relate genetic variation in GABA-genes, BDNF, ER, AR, and GR genes to these sex differences in brain abnormalities and hormonal dysregulation. There is also a postmortem study of males and females with schizophrenia and healthy controls. We will test expression levels in ER and AR mRNA, BDNF and GABA in HIPP and PFC in postmortem tissue in schizophrenia and healthy controls.
Project 3. "Electrophysiological and MRI gray matter markers and predictors of progression", Event-related potentials (ERPs) and MRI gray matter to describe progression, often conjoint, in these two domains.
- Robert McCarley, M.D., PI
- Margaret Niznikiewicz, Ph.D., Co-PI
- Cara Murphy, BS, Lead CIDAR Research Assistant
- Kevin Spencer, Ph.D., Investigator
- Rayna Zacks, BA, Research Assistant
Increasing evidence, including that from our CIDAR investigators, points to a post-onset progression of event-related potential (ERP) and MRI markers of pathology in schizophrenia, including a conjoint progression of gray matter loss in Heschlís gyrus and abnormalities of pitch mismatch negativity (MMN). However, the relative merit of each of the many putative markers in schizophrenia is not clear, while markers in the clinical high risk stage are not at all or only minimally defined. Accordingly, this project will study a selected set of putative biomarkers of vulnerability to progression in the 3 sets of participants, each with a substantial number of healthy control participants. Unifying our approach is our neurobiological model of a fundamental cellular defect in recurrent inhibition. We hypothesize that such an abnormality may be responsible for both the developmental abnormalities observed in this disorder as well as the post onset progression. This cellular-based model, and our preliminary data, has led to an increased focus on early-stage brain processing, especially auditory, as experiments in early processing appear to be especially amenable to correlation with anatomy and with documentation of progression of the disorder. One of the exciting features of this CIDAR proposal is the ability to link clinical measures of circuit abnormalities (gamma oscillations) with postmortem evidence of recurrent inhibitory abnormalities (e.g., parvalabumin neurons) and associations with genes playing a critical role in this circuitry.
We predict that clinical high risk, first episode and long-term schizophrenia groups will evince abnormalities in all ERP and associated MRI measures compared to healthy controls, and that abnormalities are more pronounced in later stages of the illness (Long-term > First Episode > Clinical high risk). We believe, since ERP variables are easier and less expensive to measure than their associated MRI variables, ERPs have advantages as biomarkers. MRI measures, including gray-white-CSF segmentation will include all temporal lobe gyri and subdivisions, and medial temporal lobe structures. We will also measure prefrontal, parietal, and occipital gyral gray matter.
Project 4. "Vulnerability to white matter progression in schizophrenia", M. Shenton, Ph.D., PI. Diffusion tensor imaging evaluations.
- Martha Shenton, PhD., PI
- Sylvain Bouix, PhD, Investigator
- Charles Davidson, BS, Research Assistant
- Hannah Fiedosewicz, MS, Recruiter
- Usman Khan, BS, Research Assistant
- Marek Kubicki, MD, PhD, Investigator
- Yogeshi Rathi, PhD, Post-Doctoral Fellow
- Lucas Torres, M.D.. Research Fellow
While a great deal of progress has been made in identifying and delineating gray matter abnormalities in schizophrenia, it has been only recently that the same level of scrutiny has been applied to investigating white matter abnormalities in schizophrenia. Moreover, recent post-mortem studies indicate that astrocytes and oligodendrocytes, glial cells involved in signal conduction and cell metabolism, are abnormal in schizophrenia, and since glial cells also play an important role in neuronal migration and in synaptic function (including glutamatergic/NMDA regulation), their dysfunction could lead to reduced neuronal size, reduced levels of synaptic proteins, as well as to abnormalities in neurotransmission and functional dysconnectivity. Of further note, recent genetic studies also point to oligodendrocyte and myelin related (OMR) genetic abnormalities in schizophrenia. Taken together, these findings suggest that the current Diffusion Tensor Imaging study to evaluate white matter, including changes over time, and their association with variations in OMR genes and cognitive/clinical symptoms, in clinical high risk individuals and those in the first episode, along with their status in long-term schizophrenia, may be important to furthering our knowledge of putative neurobiological markers of pathology in schizophrenia.
Cores
Important additional investigations that interface with all the projects will be made by these Cores:
The Operations and Clinical Assessment Core (L. Seidman, Ph.D., PI) is responsible for overall administration of the center grant including all subject recruitment and clinical assessments for patients and healthy volunteer controls.
- Larry Seidman, PhD, PI
- Ariel Brown, MA, Neuroimaging Assistant
- Ann Cousins, MS, APRN, BC, Head of Training for Clinical Ratings, Clinical Rater, Investigator
- Jean Frazier, MD, Senior Consultant on Psychosis in Adolescents and Young Adults, Investigator
- Michelle Friedman-Yakoobian, PhD, Clinical Rater, Investigator
- Shiva Gautam, PhD, Statistician
- Lauren Gibson, Ed.M, Research Assistant, Neuropsychological Tester
- Anthony Giuliano, PhD, Prodrome Project Director, Investigator
- Andréa Gnong, MA, CIDAR Program Research Coordinator
- Nikos Makris, MD, PhD, Neuroimaging / Investigator
- Raquelle Mesholam-Gately, PhD, CIDAR Program Director, Investigator
- Corin Pilo, MA, Recruiter
- Shannon Sorenson, BA, Research Assistant, Neuropsychological Tester
- Heidi Thermenos, PhD, Neuroimaging Coordinator, Investigator
- Lynda Tucker, Data Management
- Joanne Wojcik, MS, APRN, BC, First Episode Project Director / Investigator
The Genetics Core (T. Petryshen, Ph.D., PI) is based at the Massachusetts General Hospital Center for Human Genetic Research in the Psychiatric and Neurodevelopmental Genetics Unit of the Department of Psychiatry. The Genetics Core conducts genetic analysis of DNA from all participants using advanced genotyping and analytical methods to examine the genetic contribution to schizophrenia progression and disturbances in neural circuitry.
- Tracey Petryshen, PhD, PI
- Pamela Sklar, MD, PhD, Co-PI
- Miles Nugent, Research Technician
- Shaun Purcell, PhD, Statistical Geneticist
The Imaging Core (M. Shenton, Ph.D., PI) includes structural MRI, fMRI and DTI on all subjects. The Imaging Core provides state-of-the-art MR neuroimaging techniques as well as state-of-the-art post-processing imaging tools to address important questions relevant to neuroanatomical and functional disturbances in at-risk prodromes, first episode subjects, and in chronic schizophrenia and normal control groups (for further details, please see
http://pnl.bwh.harvard.edu).
- Martha Shenton, PhD., PI
- Sylvain Bouix, PhD, Investigator
- Charles Davidson, BS, Research Assistant
- Hannah Fiedosewicz, MS, Recruiter
- Usman Khan, BS, Research Assistant
- Marek Kubicki, MD, PhD, Investigator
- Yogeshi Rathi, PhD, Post-Doctoral Fellow
- Lucas Torres, M.D.. Research Fellow
The Post Mortem Core (T.-U. W. Woo, M.D., Ph.D., PI) examines the possible alteration in the expression of genes of interest in specific subsets of glutamatergic and GABAergic neurons in schizophrenia using existing postmortem brain specimens from the Harvard Brain Tissue Resource Center at McLean Hospital and, together with the Genetics Core, links the genetic association of progression indices determined from each of the constituent projects to disturbances of neural circuits in the cerebral cortex. We hypothesize that genes and neural circuits that mediate neuronal oscillations in the gamma frequency band are involved in disease progression during the early course of illness. Hence, identification of these genetic and cellular elements will provide insight into novel, neurobiologically-informed therapeutic targets.
- T.-U. Wilson Woo, M.D., Ph.D., PI
- Maribel Lim, B.S., Research Assistant
- Charmaine Pietersen, Ph.D., Post-doctoral Fellow

