Tsung-Ung W. Woo, MD, PhD

Biography
Dr. Woo is a physician/scientist who completed his residency training in psychiatry at the UCLA Neuropsychiatric Institute and Hospital, research fellowship training in the laboratory of David A. Lewis, M.D. at the Western Psychiatric Institute and Clinic of the University of Pittsburgh Medical Center and clinical research training with Alan I. Green, M.D. at Harvard Medical School. He is currently Assistant Professor of Psychiatry at Harvard Medical School. He holds appointment at both the Beth Israel Deaconess Medical Center and McLean Hospital.Research Summary
Dr. Woo's main interest is in trying to understand how neural circuits in the cerebral cortex may be altered in psychotic disorders, such as schizophrenia and bipolar disorder. He is particularly interested in finding out how subsets of inhibitory neurons in the cerebral cortex, which regulate different aspects of information flow, may be differentially disturbed in these disorders. His laboratory has three major focuses of investigation:Postmortem Human Brain Studies
Using advanced molecular, genetic and genomic techniques, alterations in gene expression in specific neuronal populations in the cerebral cortex in schizophrenia and bipolar disorder are being examined. So far, the evidence available seems to suggest that a specific subset of inhibitory neurons that contain the calcium-binding protein parvalbumin, which provide perisomatic (basket cells) and axo-axonic (chandelier cells) inhibition to pyramidal neurons, may be preferentially disturbed in schizophrenia. These cells are critical for the generation of brain oscillations in the gamma frequency band, which are believed to be deficient in patients in schizophrenia. Our working hypothesis is that disturbances of the maturation of these neurons during the period of late adolescence and early adulthood may play a role in triggering the onset and progression of schizophrenia.
Monkey Brain Development
Using similar techniques, the goal of this research is to try to understand how the disturbances of neural circuits in the cerebral cortex in schizophrenia arise during development, especially during the critical period of late adolescence and early adulthood, when schizophrenia symptoms and deficits typically begin to clinically manifest.
Clinical Translational Research
The goal of this line of work is to test hypotheses about the developmental pathophysiology of schizophrenia that are generated from our postmortem human brain and monkey brain development studies in the clinic. Thus, we want to find out if pharmacologic interventions that target specific aspects of the postulated pathophysiologic process may be effective in attenuating or perhaps even reversing the functional deterioration that occurs during the early course of schizophrenia.
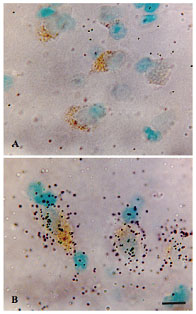
A. Inhibitory neurons expressing the mRNA for glutamic decarboxylase (GAD1), labeled with digoxigenin. B. Digoxigenin-labeled neurons that also express the mRNA for the NR2A subunit of the N-methyl-D-aspartate (NMDA) receptor, labeled with 35S autoradiography.
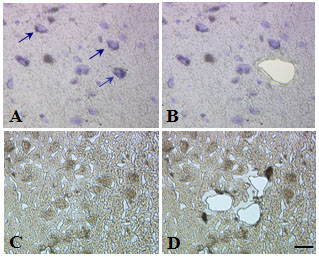
Isolation of individual pyramidal cells (A, B) and parvalbumin-immunoreactive nueonrs (C, D) using laser capture microdissection.
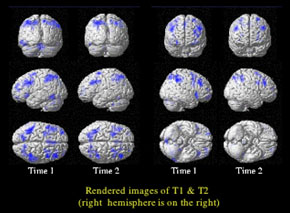
Functional magnetic resonance imaging study showing normalization of prefrontal cortical activation during working memory in a 21 year-old patient, recently diagnosed schizophrenia, after treatment with a compound that targets the inhibitory circuitry (Time 2) compared to baseline (Time 1).
Selected Publications
- Woo TUW, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM (2007) Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophrenia Research 96:46-61.
- Woo TUW, Crowell AL (2005) Targeting synapses and myelin in the prevention of schizophrenia. Schizophrenia Research 73: 193-207.
- Woo TUW, Walsh JP, Benes FM (2004) Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of General Psychiatry 61: 649-657.
- Woo TUW, Whitehead RE, and Lewis DA (1998) Axon terminals of chandelier neurons are selectively altered in the prefrontal cortex in schizophrenia. Proceedings of the National Academy of Sciences USA 95: 5341-5346.
- Woo TUW, Pucak ML, Kye CH, Matus CV, and Lewis DA (1997) Peripubertal refinement of intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience 80: 1149-1158.
