Anthony Rosenzweig, M.D.

Contact
arosenzw @ bidmc.harvard.eduBiography
Dr. Rosenzweig received his undergraduate degree from Harvard College magna cum laude in biochemistry and molecular biology in 1979 and then attended Harvard Medical School where he graduated in 1984. After clinical training in medicine and cardiology at the Massachusetts General Hospital (MGH), Dr. Rosenzweig trained in molecular biology and genetics in the laboratory of Drs. Jonathan and Christine Seidman -- both investigators in this Leducq Network -- in the Department of Genetics at Harvard Medical School from 1988 to 1991. Between 1991 and 1994, Dr. Rosenzweig undertook additional training in cell biology and experimental pathology with Dr. Michael A. Gimbrone, Jr, in the Vascular Research Division of the Brigham and Women's Hospital. From 1994 to 2005, Dr. Rosenzweig was an independent investigator in the MGH Cardiovascular Research Center (CVRC). In 2006, Dr. Rosenzweig became the Director of Cardiovascular Research and Associate Chief of Cardiology at the Beth Israel Deaconess Medical Center (BIDMC). In addition to serving as the American Coordinator for the CarDiaNet Leducq Network, Dr. Rosenzweig serves as a member of the Harvard Stem Cell Institute Executive Board and an Associate Editor of the New England Journal of Medicine.Research Summary
The Rosenzweig laboratory has focused on the use of genetic models -- both through somatic gene transfer and germline manipulation -- to address questions relevant to the two most common forms of heart disease: heart failure and atherosclerotic vascular disease. In both settings, research has focused on signaling mechanisms important to the pathophysiology of these conditions. Dr. Rosenzweig's laboratory was the first to use somatic gene transfer to create models of heart failure and to document the feasibility of manipulating global ventricular function through in vivo cardiac gene transfer (PNAS, 1998). A particular focus has been pathways controlling cardiomyocyte survival and growth. These studies demonstrate a critical role for PI 3-kinase and Akt signaling pathways both in vitro and in vivo (Circulation, 1999, 2001, JBC 2002a) but also found that chronic activation of Akt (as seen in heart failure patients) can have adverse consequences due to feedback inhibition of upstream signaling (JCI, 2005) thus underscoring the importance of Akt-independent mechanisms. The laboratory has gone on to identify an important role of Akt-independent pathways such as SGK1 in the heart (Circulation, 2005) using a combination of traditional biochemical approaches and high throughput screens, such as transcript profiling. A growing interest in the laboratory is the intersection of these pro-survival signaling pathways with those controlling cardiac metabolism (AJP, 2006) and this interest prompted collaborative projects with the Cook and Spiegelman laboratories (JBC, 2002; Cell Metabolism, 2005; PNAS, 2006) that helped stimulate the formation of this CarDiaNet international LeDucq Foundation Network of Research Excellence.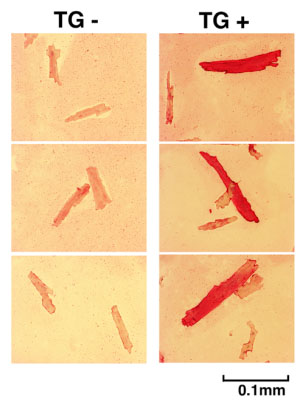
Cardiomyocytes isolated from chimeric hearts of X-linked Akt1 transgenics (right panels) compared to those from littermate controls. Transgene (stained red) expressing cardiomyocytes are larger than non-expressing cardiomyocytes isolated from the same hearts.
Publications
- Hajjar RJ, Schmidt S, Matsui T, Guerrero L, Lee K-H, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proceedings of the National Academy of Sciences (USA), 1998;95:5251-5256.
- Gerszten RE, Garcia-Zepeda E, Lim Y-C, Yoshida M, Ding H, Gimbrone MA, Luster A, Luscinskas FW, Rosenzweig A. The chemokines MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999;398:718-23.
- Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, FrankeT, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001;104:330-335.
- Nagoshi, T., Matsui, T., Aoyama, T., Leri, A., Anversa, P., Li, L., Ogawa, W., Del Monte, F., Gwathmey, J.K., Grazette, L., Hemmings B, Kass D, Champion H, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. Journal of Clinical Investigation 2005;115:2128-2138.
- Morissette, M.R., Cook, S.A., Foo, S.Y., McKoy, G. Ashida, N, Novikov, N, Scherrer-Crosbie, M., Li, L., Matsui, T., Brooks, G., Rosenzweig, A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circulation Research 2006. 99(1):15-24.